Wilson disease is an autosomal recessive disease, which means it occurs equally in men and women. In order to inherit Wilson disease, both parents must carry one genetic mutation (abnormal alteration in the gene) that each parent passes to the affected child. At least one in 30,000 people of all known races and nationalities has the disease.
Of the 23 different human chromosomes, the gene responsible for Wilson disease is located on chromosome 13. The gene is called ATP7B and it contains the genetic information necessary to make a copper transport protein that plays a key role in incorporating copper into ceruloplasmin and moving excess copper out of the liver. Mutations in the gene lead to an abnormal copper transporter that cannot move copper effectively or at all. More than 300 genes of the ATB7B have been identified thus far. This excess copper accumulates in the liver and other organs.
Most patients have no family history of Wilson disease. People with only one abnormal gene are called carriers. Carriers (heterozygotes) may have mild, but medically insignificant, abnormalities of copper metabolism. Carriers do not become ill and should not be treated. Wilson disease patients (homozygotes) do become ill and must receive treatment lifelong or eventually they will develop severe lethal disease.
What is the likelihood of inheriting Wilson disease?
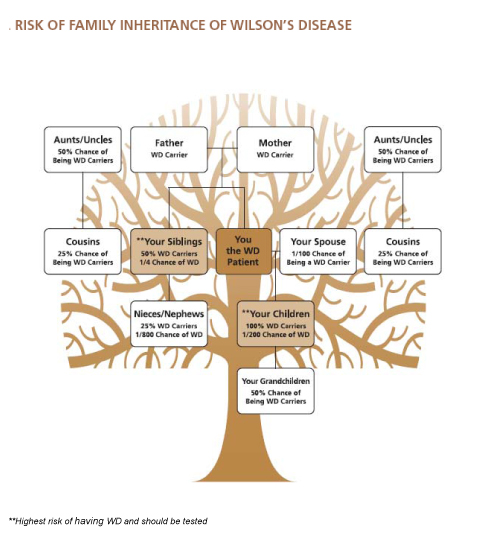
One in 100 individuals in the general population carries one abnormal copy of the Wilson disease gene. Carriers have one normal and one abnormal gene. All (100%) children of those afflicted with Wilson disease receive at least one abnormal copy of the Wilson disease gene. One half (50%) of a carrier's children receive at least one abnormal copy of the Wilson disease gene. This diagram is a basic risk profile for family members. A genetic counselor can provide a more detailed pedigree of specific family relationships.
All siblings and children of Wilson disease patients should be tested for Wilson disease. Other relatives who have had symptoms or laboratory tests that indicate liver or neurological disease also should be tested for Wilson disease.

29 Aug 2018
Complete Prescribing Information for Trientine as a printable PDF...

10 Sept 2018
Get answers to questions about how Trientine works, and more...
“Treatment of patients with Trientine Hydrochloride Capsules may be complicated by severe, sometimes lifethreatening, adverse effects. Trientine Hydrochloride Capsules should be administered under the supervision of a physician experienced in the use of this medication for the treatment of sickle cell anemia.”
“Hydroxyurea is mutagenic and clastogenic, and causes cellular transformation to a tumorigenic phenotype. Hydroxyurea is thus unequivocally genotoxic and a presumed transspecies carcinogen which implies a carcinogenic risk to humans. In patients receiving long-term hydroxyurea for myeloproliferative disorders, such as polycythemia vera and thrombocythemia, secondary leukaemia’s have been reported. It is unknown whether this leukemogenic effect is secondary to hydroxyurea or is associated with the patient's underlying disease. The physician and patient must very carefully consider the potential benefits of Trientine Hydrochloride Capsules relative to the undefined risk of developing secondary malignancies. Trientine Hydrochloride Capsules is used to treat chronic myeloid leukaemia or cervical cancer.”
“Your medical team will discuss with you the options for treating your cancer. They will take into account factors such as the type of cancer, where it is, which stage it is at and whether you have had treatment before. The results of blood tests and other investigations will also be considered. How well you feel and how you are likely to cope with treatment is also important.”
“Your cancer treatment will usually consist of a treatment session with Trientine Hydrochloride Capsules followed by a break of a number of days before the next treatment session with Trientine Hydrochloride Capsules. This cycle may be repeated many times as part of your cancer treatment. Trientine Hydrochloride Capsules works by damaging cancer cells in the body. Trientine Hydrochloride Capsules also affects healthy cells and treatment with Trientine Hydrochloride Capsules may damage your immune system. Your medical team may arrange for you to have some blood tests to check how well your immune system is working. Do not share your medicine with other people. It may not be suitable for them and may harm them. The pharmacy label on your medicine tells you how much medicine you should take. It also tells you how often you should take your medicine. This is the dose that you and your prescriber have agreed you should take. You should not change the dose of your medicine unless you are told to do so by your prescriber. If you feel that the medicine is making you unwell or you do not think it is working, then talk to your prescriber.